How to Determine Which Indicator to Use for Titration
EBT is the indicator used in the determination of hardness. An acidbase indicator eg phenolphthalein changes color depending on the pH.

18 3 Identify An Appropriate Indicator For A Titration Hl Ib Chemistry Youtube
Now you might think that when you add an acid the hydrogen ion would be picked up by the negatively charged oxygen.

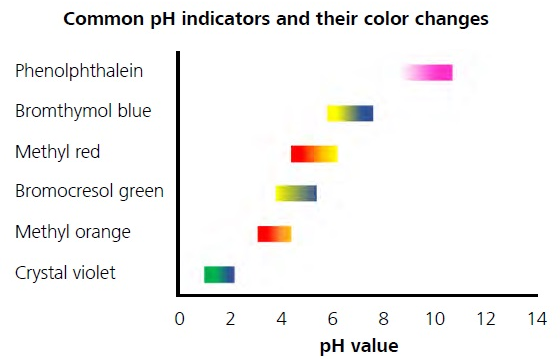
. Colour in acidic solution. Universal indicator which is actually a mixture of several indicators displays a variety of colours over a wide pH range so it can be used to determine an approximate pH of a solution but is not used for titrations. 1 pH range of the indicator cuts the.
Molarity is the concentration of a solution expressed as the number of moles of solute per litre of solution. Thus the indicators like methyl orange methyl red and phenolphthalein can show the colour change in the ph range of 4t0 10. What factors govern the choice of a suitable indicator for an acid base titration.
Fehlings reagent refers to the mix of 10 mL each of stock solutions A and B 8. In an alkaline solution methyl orange is yellow and the structure is. Thus in strong acid- strong base titrations any one of the above indicators can be used.
Modified 2 years 5 months ago. Use a table of indicator colour and pH range to choose an indicator which changes. It is usually assumed that at pH pKa 1 the acid form dominates whereas at pH pKa 1 the basic form dominates.
A You may be told the pH of the solution eg in an exam. Commonly used indicators are phenolphthalein and methyl orange. Ethylenediaminetetraacetic acid EDTA is used to determine the hardness of water with the help of complexometric titration.
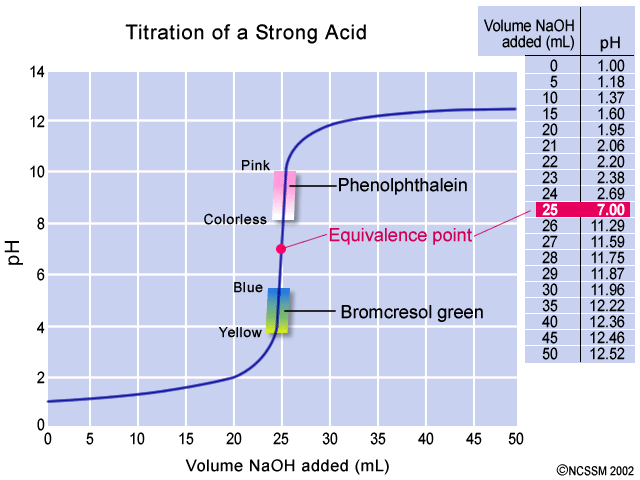
A strong acid- strong base titration isperformed usinga phenolphthalein indicator. 1 The equivalence point of an acid-base reaction the point at which the amounts of acid and of base are just sufficient to cause complete neutralization. We will use formula derived in the acid-base titration curve calculation section.
In a strong acid-weak base titration the pH is less than 7 at the equivalence point. To identify the equivalence point in the titration we use titration curves and indicatorsAccording to the concentration of acid and base solutions we have to choose correct curve and indicator. Use the titration formula.
Phenolphthalein a commonly used indicator in acid and base titration. It is the pH indicator commonly used in titration. A drop of indicator solution is added to the titration at the beginning.
Titration Experiment Calculate the Molarity of Acetic Acid in Vinegar. For example in the titration of a strong acid with a strong base the. Methyl orange changes color at the pH of mid strength acid.
If the titrant and analyte have a 11 mole ratio the formula is molarity M of the acid x volume V of the acid molarity M of the base x volume V of the base. How can you use indicator titration to determine the pH of a complex solution like ocean water if at all. Redox indicators are also used.
When selecting an indicator for acid-base titrations choose an indicator whose pH range falls within the pH change of the reaction. For strong acids and weak bases methyl orange is the indicator of choice. When silver nitrate is slowly added the precipitate of silver chloride is formed in the solution.
Use the titration formula. If the titrant and analyte have a 11 mole ratio the formula is molarity M of the acid x volume V of the acid molarity M of the base x volume V of the base. It will appear pink in basic solutions and clear in acidic solutions.
It isknown as the titrant. Colour in alkaline solution. In alkaline solution methyl orange is in yellow color.
That for known pH value allows easy calculation of a volume of the titrant strong base in this case that was added added to a strong acid. 50 mL of 01M strong acid titrated with 01 M strong base. Determine the moles of sodium chloride according to calculating the moles of reacted silver nitrate after finishing titration.
This information is given by the pKa of the acid-base couple. Solution used for the titration determine the mass of dextrose that reacts per mL of Fehlings reagent. It is also very common indicator a weak acid used in titration.
NaOH is a strong alkali and HCl acid is a strong acid respectively. The full form of EBT is Eriochrome Black T. The analyte which is the volatile substance is first allowed to react with the excess reagent.
In this way how do you choose an indicator for an acid base titration. A titration is then performed on the remaining amount of the known solution to determine how much is in excess and to measure the quantity consumed by the analyte. Can you use universal indicator for titration.
There are two steps in deciding which indicator to use for a particular acid-base titration. Viewed 788 times 0 begingroup Could you measure the pH of ocean water by using a strong acid-weak base titration. Phenolphtalein ischosen because it changes color in a pH range between 83 10.
PH indicators - color changes pH and titrant volumes required. Repeat the titration at least three times. But in this case instead of one indicator two indicators are used because there are going to be two endpoints during the titration.
Determine the pH of the solution at the equivalence point. Ask Question Asked 2 years 5 months ago. The end point of the titration occurs when all the chloride ions are precipitated and an indicator will indicate at once.
2 The pH of the solution at equivalence point is dependent on the strength of the acid and strength of the base used in the titration. An indicator is suitable only if it undergoes a change of colour at the pH near the end point. Click to see full answer.
The general shape of the titration curve is the same but the pH at the equivalence point is different. A back titration is normally done using a two-step procedure. The end point of an acid-base titration can be detected by the use of a pH indicator a pH meter or a conductance meter.
Titration curve of weak acid and strong base. When acid is added in the solution it gives red color. Colour in neutral solution.
What indicator is used in titration. Methyl orange is one of the indicators commonly used in titrations. For finding the composition of the mixture or say to check the purity of a sample titration of the mixture is done against a strong acid.
Weak Acid against Strong Base. In a weak acid-strong base titration the pH is greater than 7 at the equivalence point. The correct answer is EBT.
The endpoint has been reached when the color changes. Therefore to have the colour change when the equivalence is reached the pH then must be as close as possible of the pKa of the indicator. Extra dextrose solution may be disposed of in the sink.
The use of a pH meter to quantitatively determine the end point of a titration reaction is preferred to the use of visual indicator.
Automated Vs Manual Titration Which Should You Use

General Chemistry Online Faq Acids And Bases How Can Strong And Weak Acids Be Distinguished Using Indicators

Titration Design Of The Experiment Medsolut

Which One Is The Best Indicator For Acid And Base Titration Between Methyl Orange And Phenolphthalein Quora
How Do We Determine The Right Indicator For An Acid Base Titration Do We Look At The Ph At The Equivalence Point Please Provide Examples As Well Quora

Learning Goal 27 Explain What Acid Base Indicators Are And How They Work Certain Chemicals Have The Special Property Of Appearing One Colour When In A Solution Of One Ph And A Different Colour When In A Solution Of A Different Ph Such Chemicals Are

22 How To Choose An Indicator Youtube

Acid Base Indicators Introduction To Chemistry
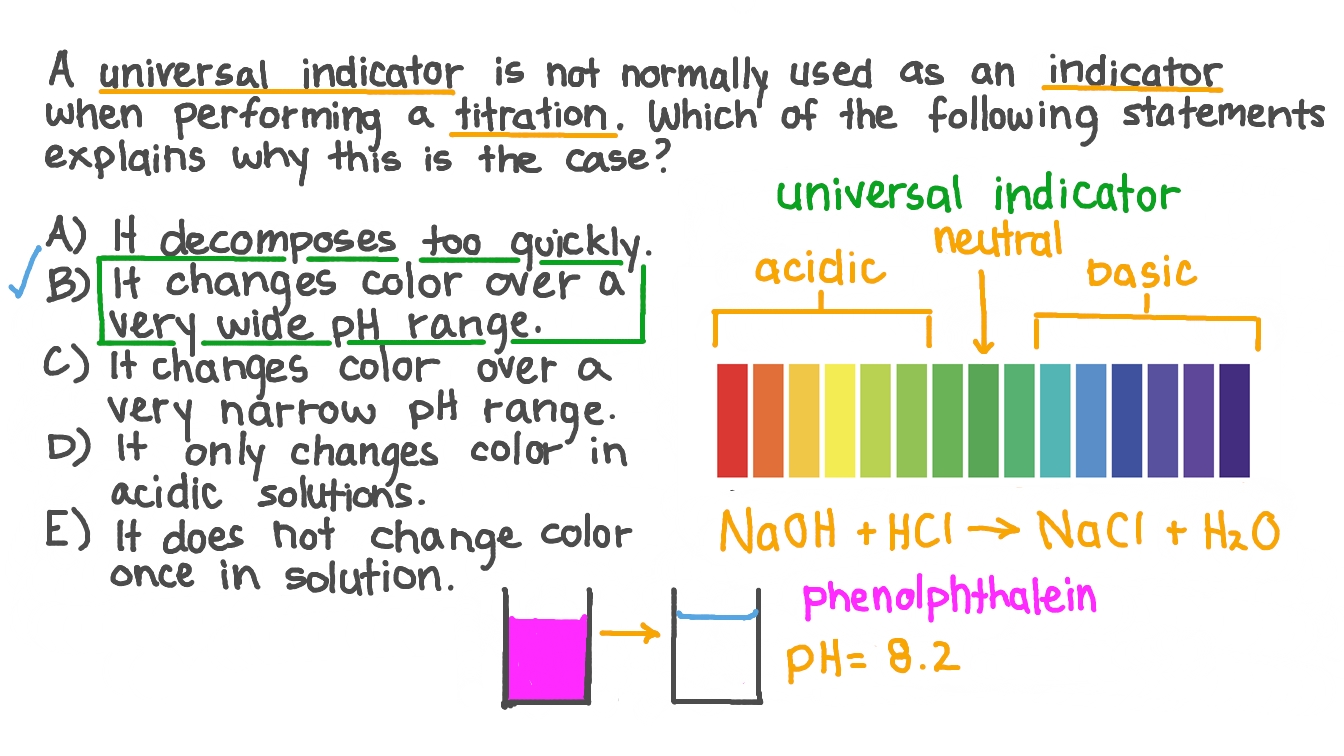
Question Video Understanding Why The Universal Indicator Is Not An Appropriate Indicator For Titration Experiments Nagwa

Acid Base Indicators Carolina Com

Titration Curves And Acid Base Indicators Chemistry Khan Academy Youtube
In Titration What Are The Different Types Of Indicators Quora

Acid Base Titration Titration Curves Equivalence Point Indicators Of Acid Base Titration

Indicators Titration Mcat Content
Chemistry Volumetric Analysis Indicators For Titrations

Acid Base Titrations Chemistry For Majors
Comments
Post a Comment